Our team is developing drug candidates that primarily focus on de-risked, clinically validated targets—biological pathways already shown to play a key role across a range of conditions. By building on proven scientific insights, we aim to translate this knowledge into therapeutic strategies with a greater likelihood of success and meaningful impact for patients.
LPA1R Antagonists
A Potential Key to Arrest Inflammatory & Fibrotic Disease Processes
LPA1R Antagonists in Inflammatory & Fibrotic Diseases
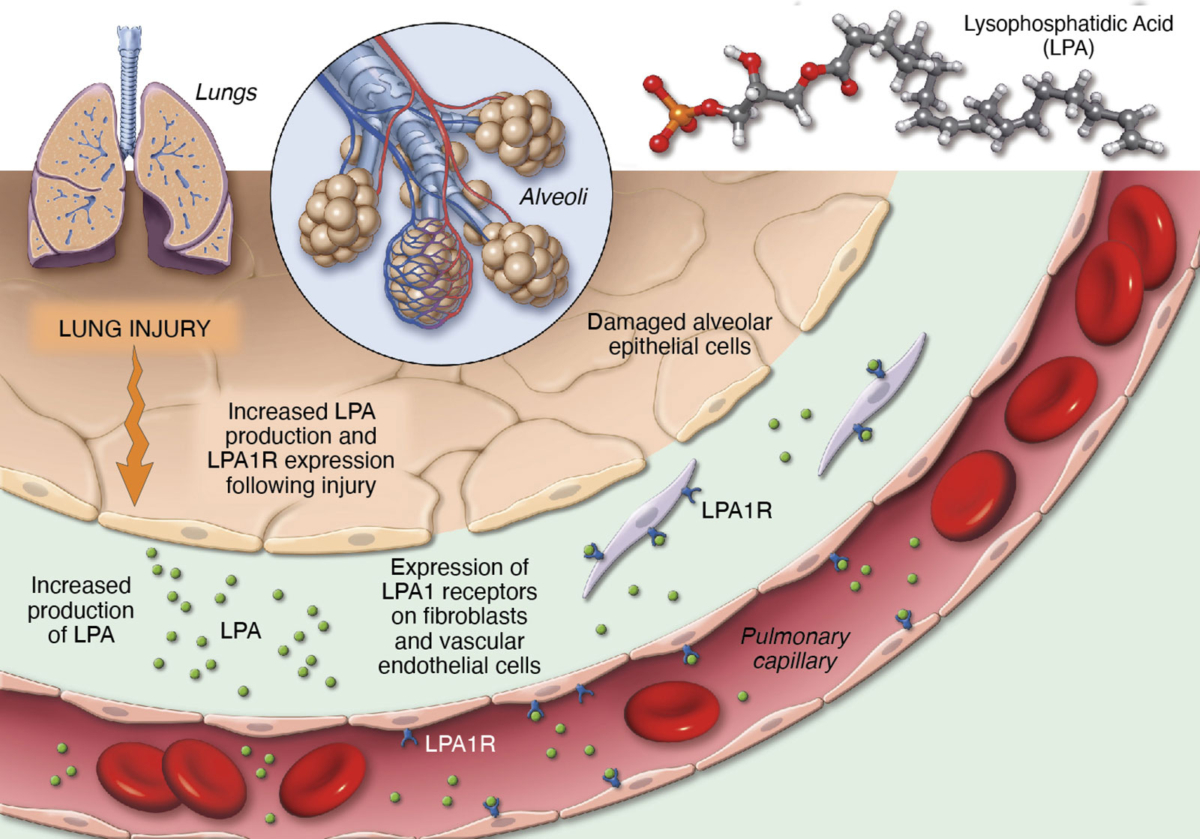
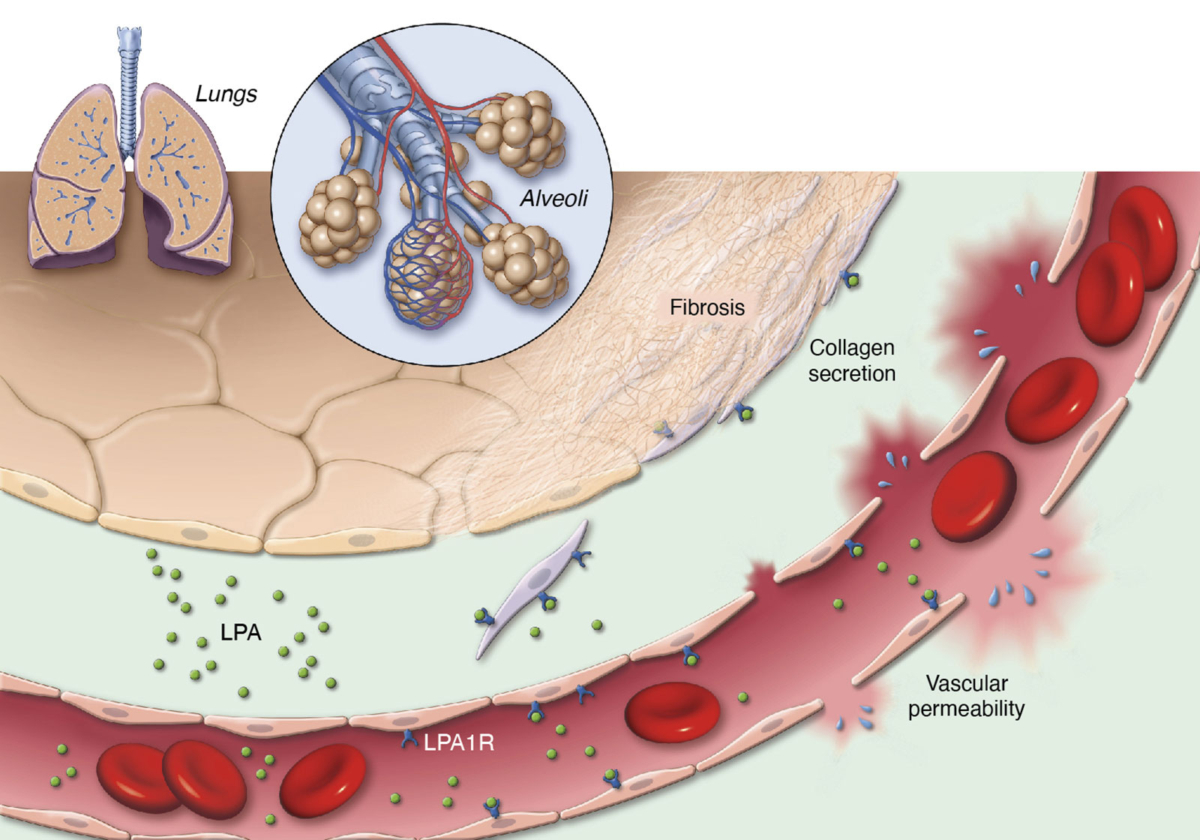
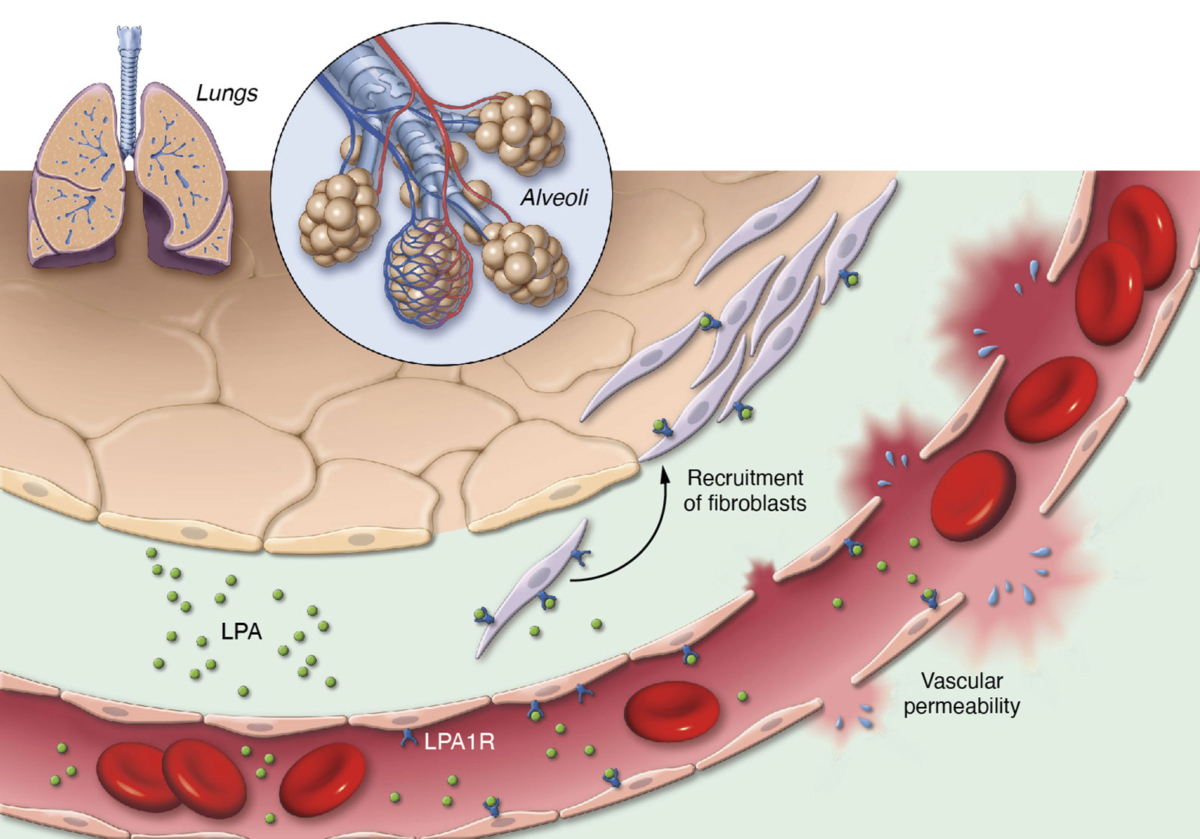
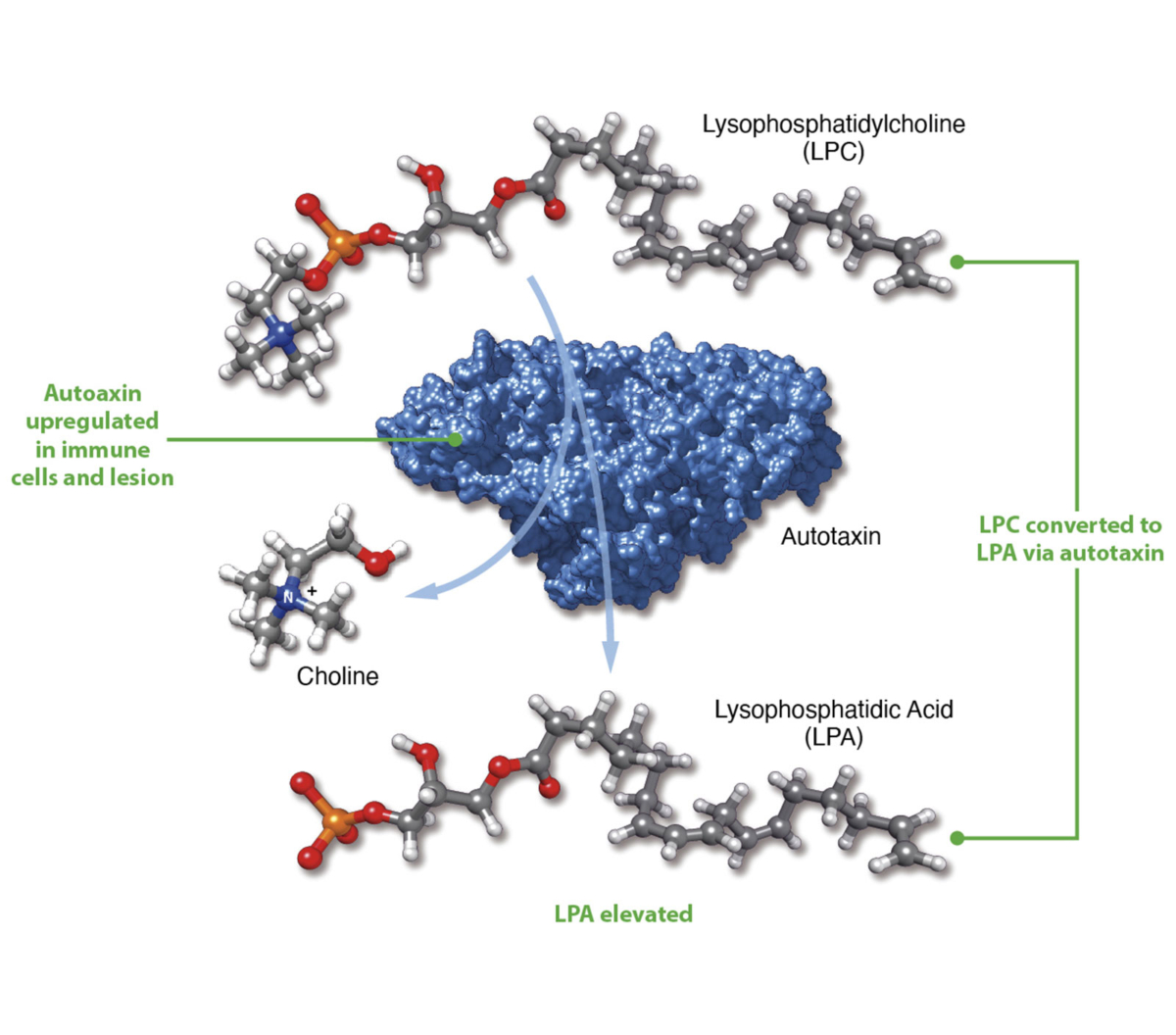
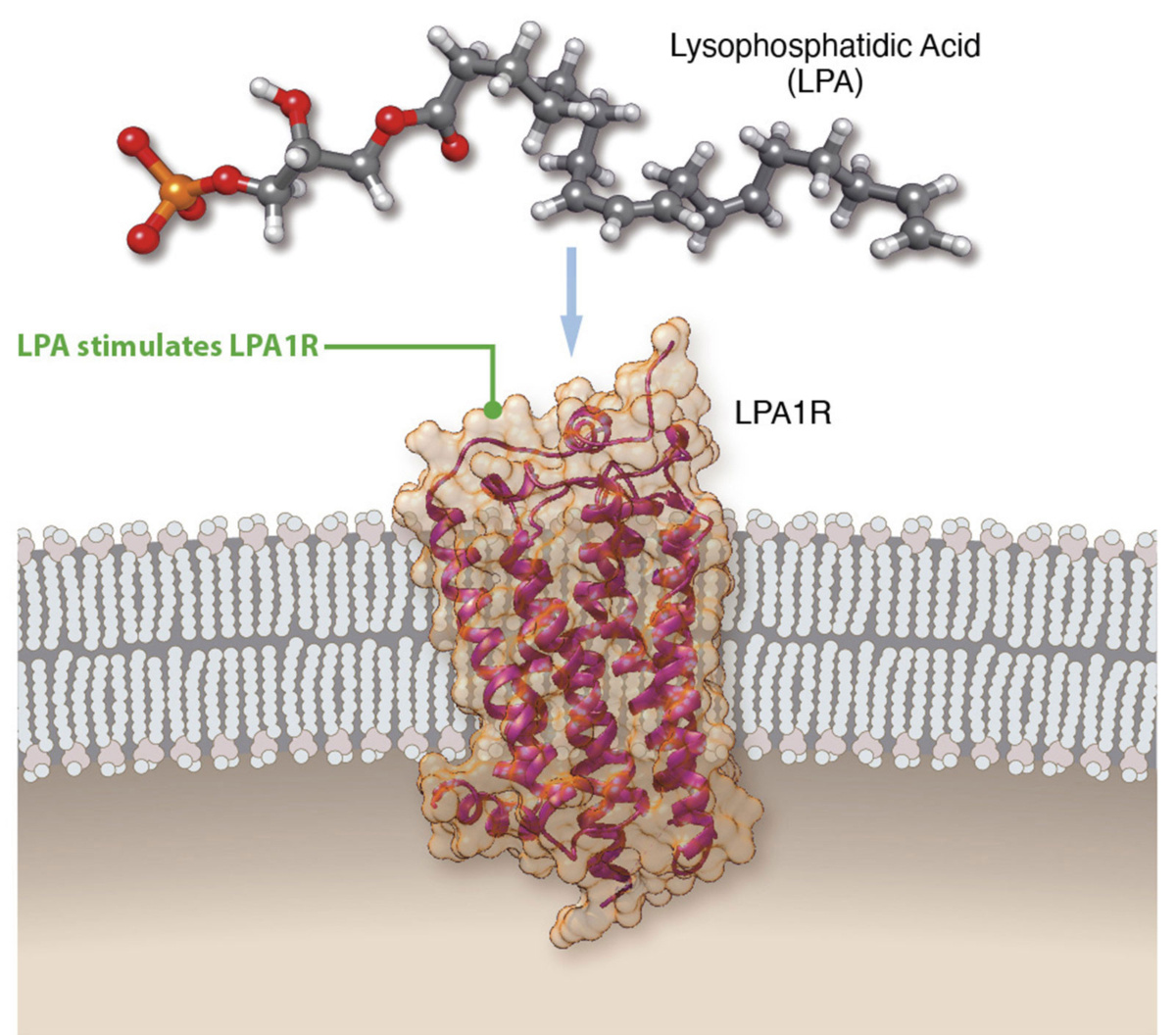
Lysophosphatidic acid (LPA) is a pro-inflammatory lipid that regulates numerous cellular functions and is also recognized as a novel mediator of wound healing and tissue fibrosis. LPA signals through a family of six G protein-coupled receptors – LPA1 to LPA6. The LPA1 receptor specifically plays a central role in several inflammatory and fibrotic diseases.
We are advancing our LPA1R antagonist, PIPE-791, as a best-in-class therapy in idiopathic pulmonary fibrosis (IPF).
Blocking LPA1R inhibits key steps in the fibrosis pathway
Hover on images to zoom
LPA1R Antagonists in Multiple Sclerosis (MS)
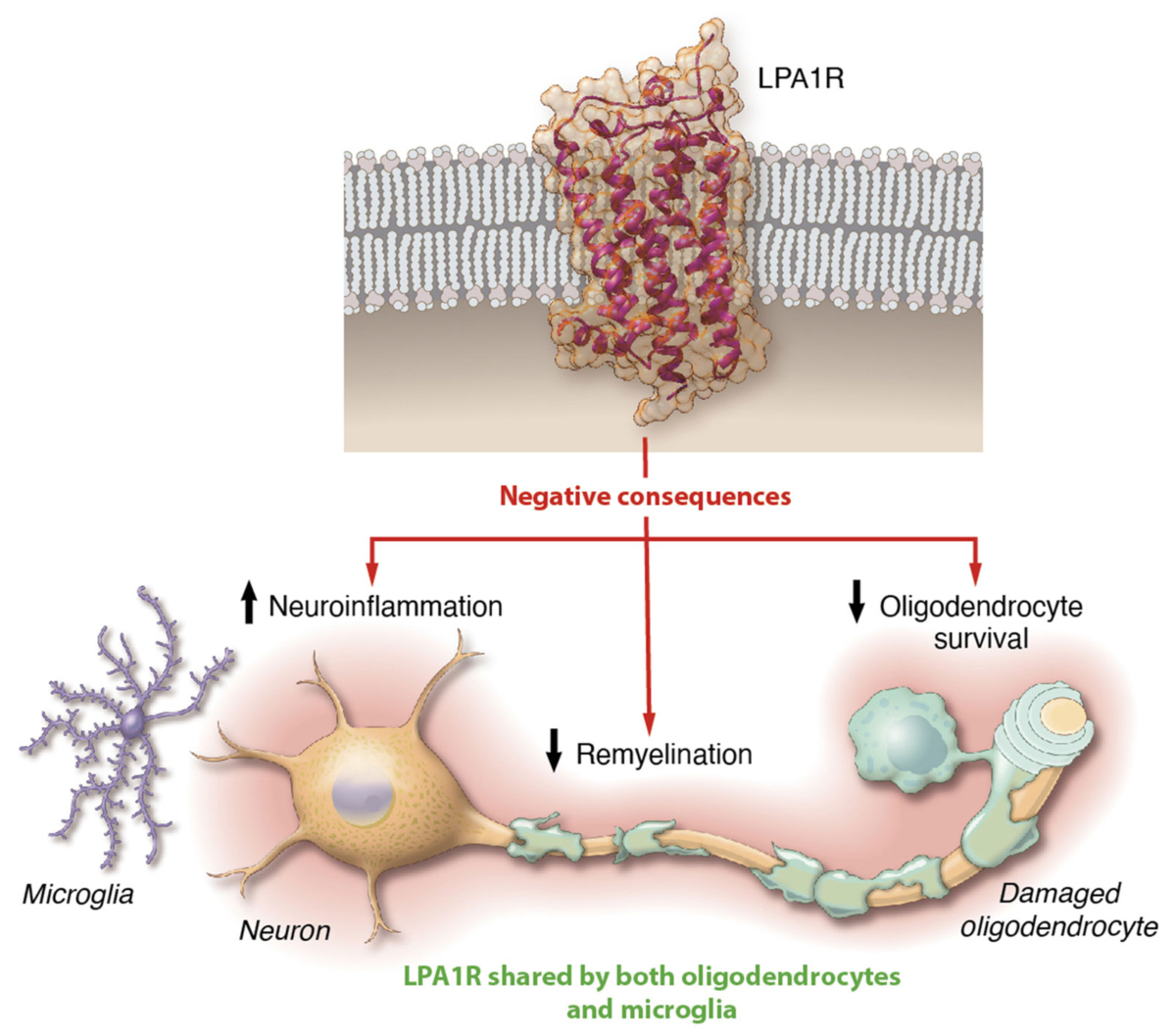
In addition to fibrotic diseases, LPA is also elevated in the plasma and cerebrospinal fluid of MS patients. LPA may promote neuroinflammation and limit remyelination—the process of damaged myelin sheaths becoming restored—by activating specific receptors such as LPA1R. Blocking the LPA/LPA1R pathway has the potential to be disease-modifying by reducing neuroinflammation and promoting remyelination.
LPA1R is a key regulator of remyelination and neuroinflammation
Hover on images to zoom
M1R Antagonists
A Potential Way to Restore Connectivity
M1R Antagonists in Depression
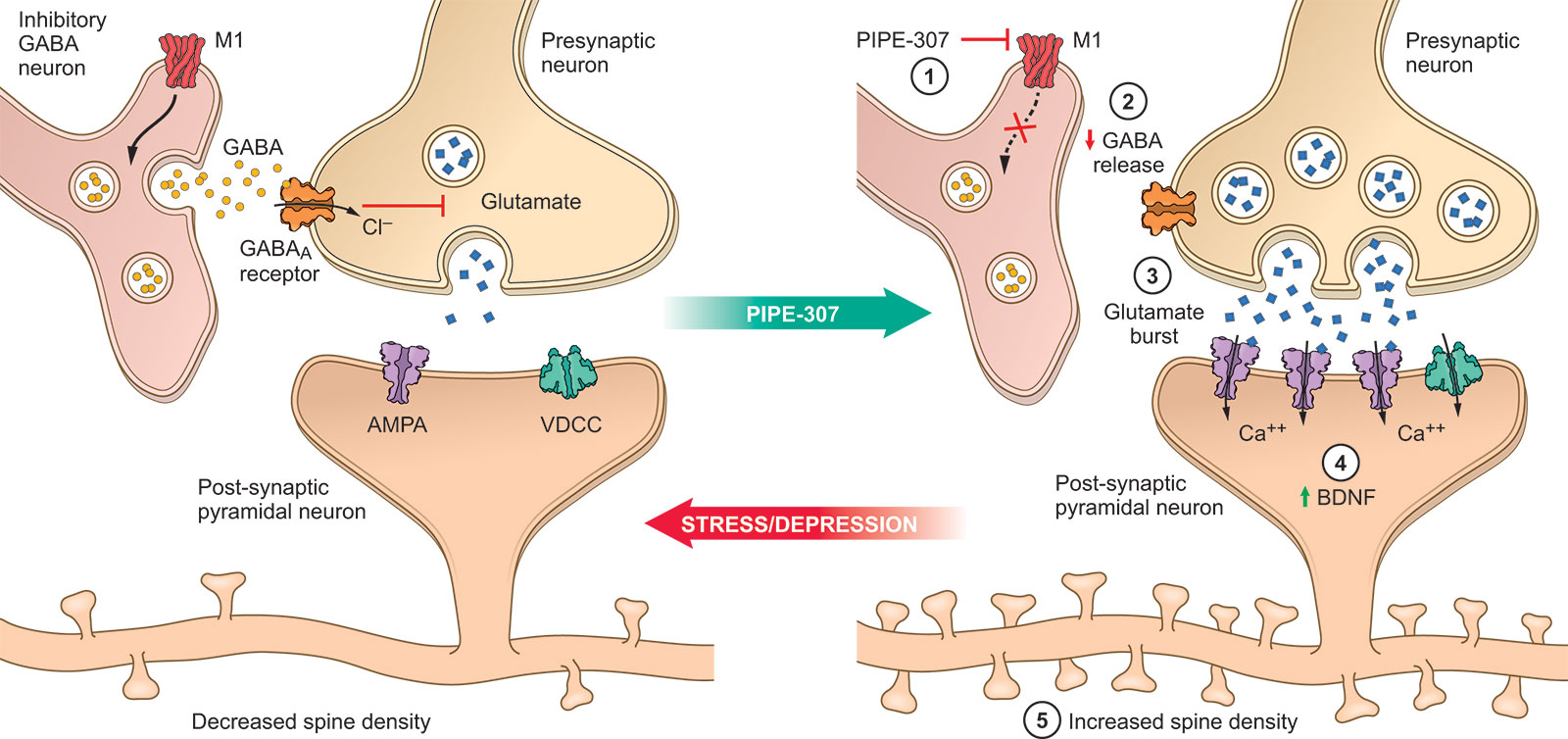
The cholinergic neurotransmitter system was first implicated in the pathophysiology of mood disorders in the 1970s. Anti-cholinergic drugs were reported to cause feelings of wellbeing in patients, and treatment with non-selective muscarinic antagonists blocked the depressive effects of physostigmine.
Our proposed mechanism involves M1 muscarinic receptor (M1R)-dependent synaptogenesis in pyramidal neurons in the prefrontal cortex. This effect is directed by blocking M1Rs located on inhibitory gamma-aminobutyric acid (GABA) neurons, which promotes excitatory transmission leading to increased brain-derived neurotrophic factor release and dendritic spine formation.
Pursuant to a licensing agreement, Janssen Pharmaceutica NV, a Johnson & Johnson company is developing our internally discovered M1R antagonist as a potential therapy in major depressive disorder.